Cart
Looks like your cart is currently empty!
Browse products
Terpene Retention & Cannabis Packaging Insights
Exploring Terpene Preservation through an experimental analysis of cannabis concentrate containers, revealing intriguing performance variations and unexpected standouts.
Stay in touch!
Join our email list to receive Calyx content & product updates.
By Tobe Nightengale in Cannabis Packaging
Abstract
The proper storage and preservation of cannabis products comes with uniquely difficult challenges, and the ability of various packaging to resist or mitigate cannabinoid and terpene degradation or loss can only be evaluated through sensitive methodology. While many standardized test methods have been developed and utilized by the pharmaceutical packaging industry, they can only approach the question of cannabis preservation, and are unsuitable for accurate shelf-life and packaging performance analysis. Each cannabis constituent requires a unique method of analysis, and Terpene Preservation is prioritized most widely across the cannabis industry.
In order to quantify the performance of Calyx Concentrate Products, and understand how our containers stack up to others in our industry, we perform a combinatory test method to accurately grade the overall performance of 11 different concentrate containers. All containers included in this test, except for Infinity Jars (Miron), are Child-Resistant containers. Non Child-Resistant Infinity Jars were utilized due to delays in obtaining CR-certified containers, and different performance may be expected in Child-Resistant Infinity Jars. This test method includes an analysis of Terpene Vapor Transmission Rate (TVTR) and Terpene Uptake (TU) over 14 Days. These methods are reported both as individual metrics, and as a combined total to display each container’s rate of Net Terpene Loss.
Final obtained data exhibits a wide range of performance across all Experimental Product Types, indicating significant difference in each container’s ability to retain and preserve Cannabis Terpenes in a packaged product. Non Child-Resistant Infinity Jars performed best in regards to Terpene Vapor Transmission Rate and Net Terpene Loss metrics, but resulted in worse performance in Terpene Uptake. This same performance cannot be guaranteed in Child-Resistant Infinity Jars. Calyx Containers (4mL and 7mL) outperformed all other Experimental Product Types in regards to Terpene Uptake, due to the superior barrier properties offered by the FEP Liner. This is also seen in the complete lack of Plastic & Gasket degradation after 14 Days of Terpene exposure. For the combined metric of Net Terpene Loss, the top performers were, in order of best performance, Non-CR Infinity Jar, Calyx 4mL, Qube 5mL, Calyx 7mL, and Qube 9mL. These metrics are accurate for the understanding of Terpene Retention & Preservation, but cannot provide direct insight into each Product Type’s ability to protect and retain Cannabinoids.
Introduction
Many container attributes and cannabis reaction pathways must be investigated in order to determine the true performance of packaging products for cannabis concentrate and flower products. While the preservation of cannabinoid profile is pivotal in determining consumption effects, the retention of Volatile Compounds & Terpenes plays a vital role in enhancing the overall quality of experience during the opening and consumption of valuable concentrates. This test method is designed to quantify the rate at which Terpenes and similar Volatiles are removed from a packaged product, either through loss to the environment or absorption into container components & materials.
These test methodologies are modeled after standard methods established by ISO & USP, regulatory agencies that act to certify laboratories, methods, and facilities for the proper manufacturing and testing of products with stringent industry requirements. Many of the methods provided by these agencies are necessary for any point in the Pharmaceutical and Biotech supply chains, and allow for the rapid and repeatable analysis of a wide variety of packaging products.
These standard methods are overwhelmingly designed for non-volatile, dry, or inorganic products, and small adjustments have been made to the methodologies to ensure that organic cannabis constituents may be properly investigated.
Utilized Equipment
OHAUS PR523/E Analytical Balance (0.001g Precision, ±0.002g Linearity, 520g Maximum Capacity)
Isolated Limonene Monoterpene Solution (USA Manufactured, GMP/ISO/FSSC22000 Compliant)
American BioTech Supply Stability Chamber (77℉, 55% RH)
Nitrile Gloves
Sterile Plastic Pipettes (3mL)
Clean Microfiber Cloth
Methodology
- Five of each container type are obtained, inspected for general damage, and thoroughly cleaned with a microfiber cloth to reduce variability of obtained data due to contamination.
- The Balance should be leveled and calibrated at the start of the test, and re-calibrated after every 5 sample measurements to ensure accuracy of obtained data.
- The Tare Weight of each experimental container should be recorded, along with the Tare Weights of the individual base and lid components.
- Each experimental container is filled to the maximum filling capacity with Isolated Limonene Solution, ensuring no spillage onto the container’s exterior.
- Each experimental container is properly sealed, and placed into a Stability Chamber, held at 77℉ for the duration of the test.
- After 7 Days (168±1 hours), experimental containers are removed from the stable environment and their new weight is recorded. Immediately after data is collected, samples are returned to the stable environment for an additional 7 Days.
- After an additional 7 Days (168±1 hours), experimental containers are again removed and their new weight recorded.
- Based on the above data, TVTR can be calculated using the following formula :
(W7 - W14)365 x 100 / (W7 - WT) x 7 = Percent per Year
- Once final weights have been obtained, each experimental container is opened and each lid component is left open to dry for 30 minutes prior to re-measurement.
- After drying in open air for 30 minutes, the lid components are weighed alone to determine any change of mass due to Terpene exposure.
- Once the final weights of the lid components have been obtained, each experimental container is washed, dried, and stored for future analysis.
- Based on the above data, TU can be calculated using the following formula :
[W(lid)]14 - [W(lid)]T / [W(VOC)]T x 100 = Percentage Uptake
- After both TVTR and TU metrics have been calculated, Net Terpene Loss can be calculated using the following formula :
((WT - W14) + ([W(lid)]14 - [W(lid)]T)) / [W(VOC)]T x 100 = Percentage Net Terpene Loss
Testing Data & Results
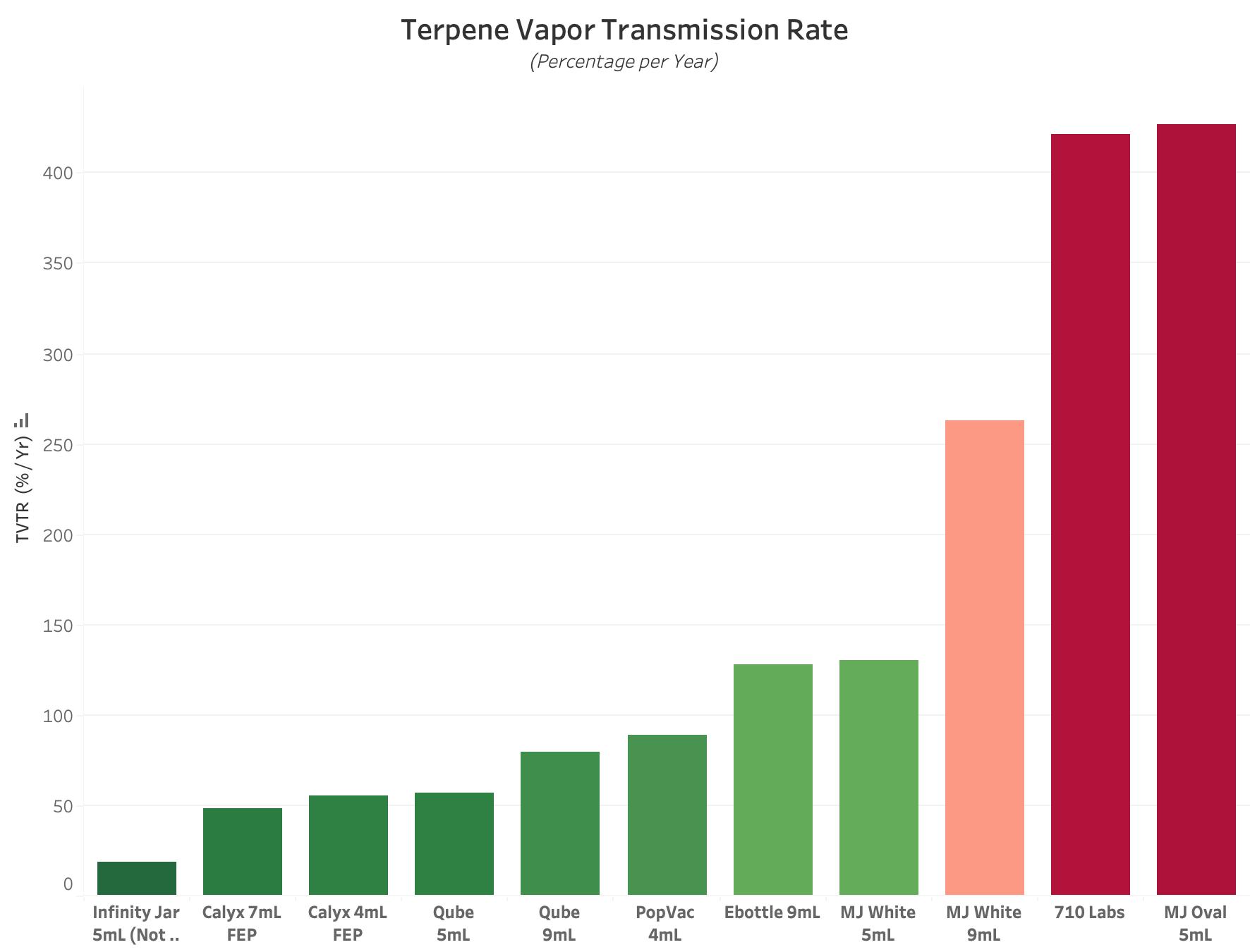
Figure 1 : Graph of Average Terpene Vapor Transmission Rate Value for each Experimental Product Type. All values are reported in units of Percentage per Year, with lower values equating to better performance. A value of 100% per Year indicates that it will take 12 Months to lose all packaged terpenes due to environmental loss.
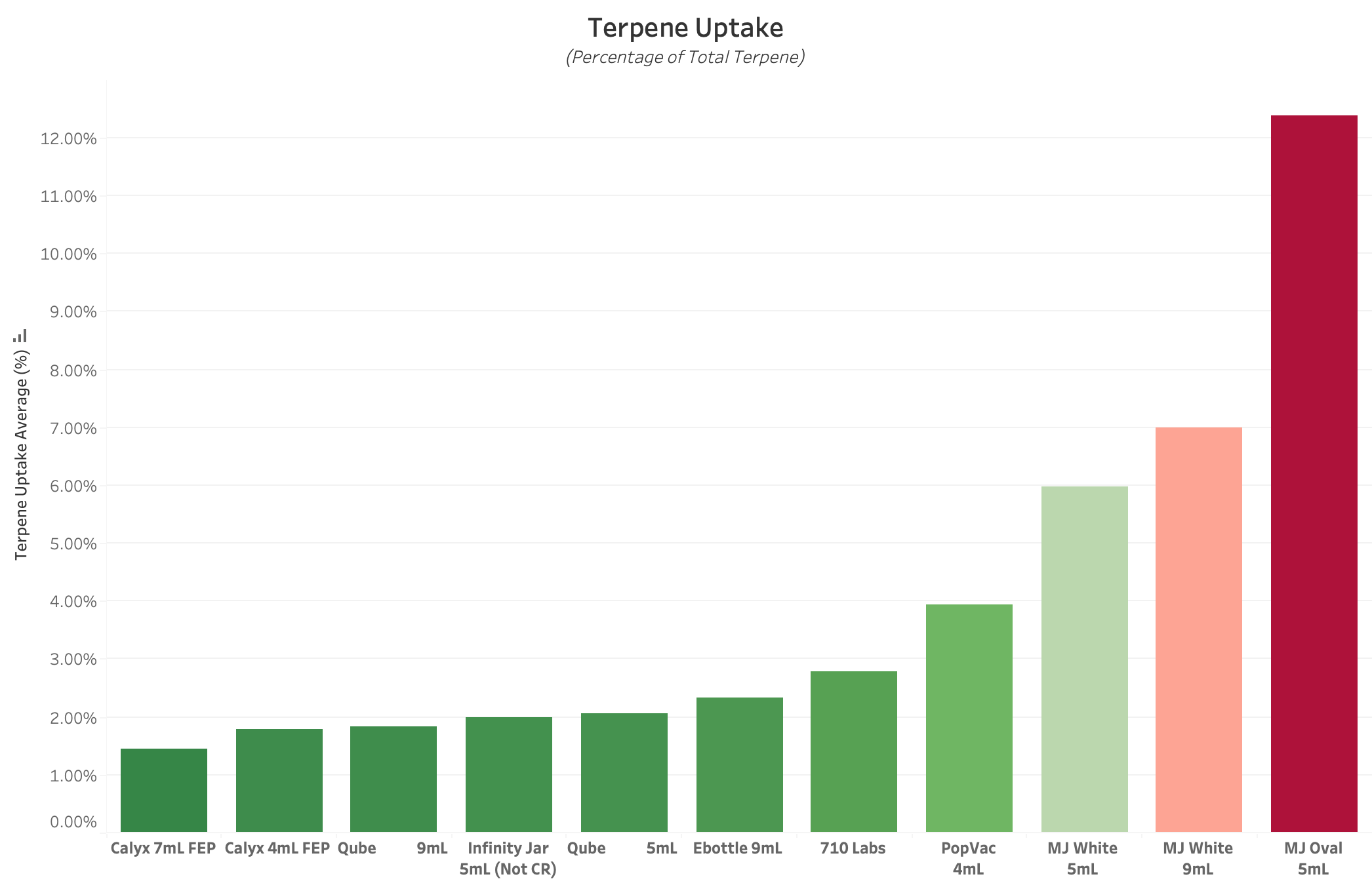
Figure 2 : Graph of Average Terpene Uptake for each Experimental Product Type. All values are reported in Percent of Total Terpene Concentration, with lower values equating to better performance. This value reflects the propensity of each container’s non-glass components to absorb and sequester Terpenes and other VOCs from packaged product over 14 Days. Plastics and Rubbers typically exhibit the highest rate of Terpene Uptake.
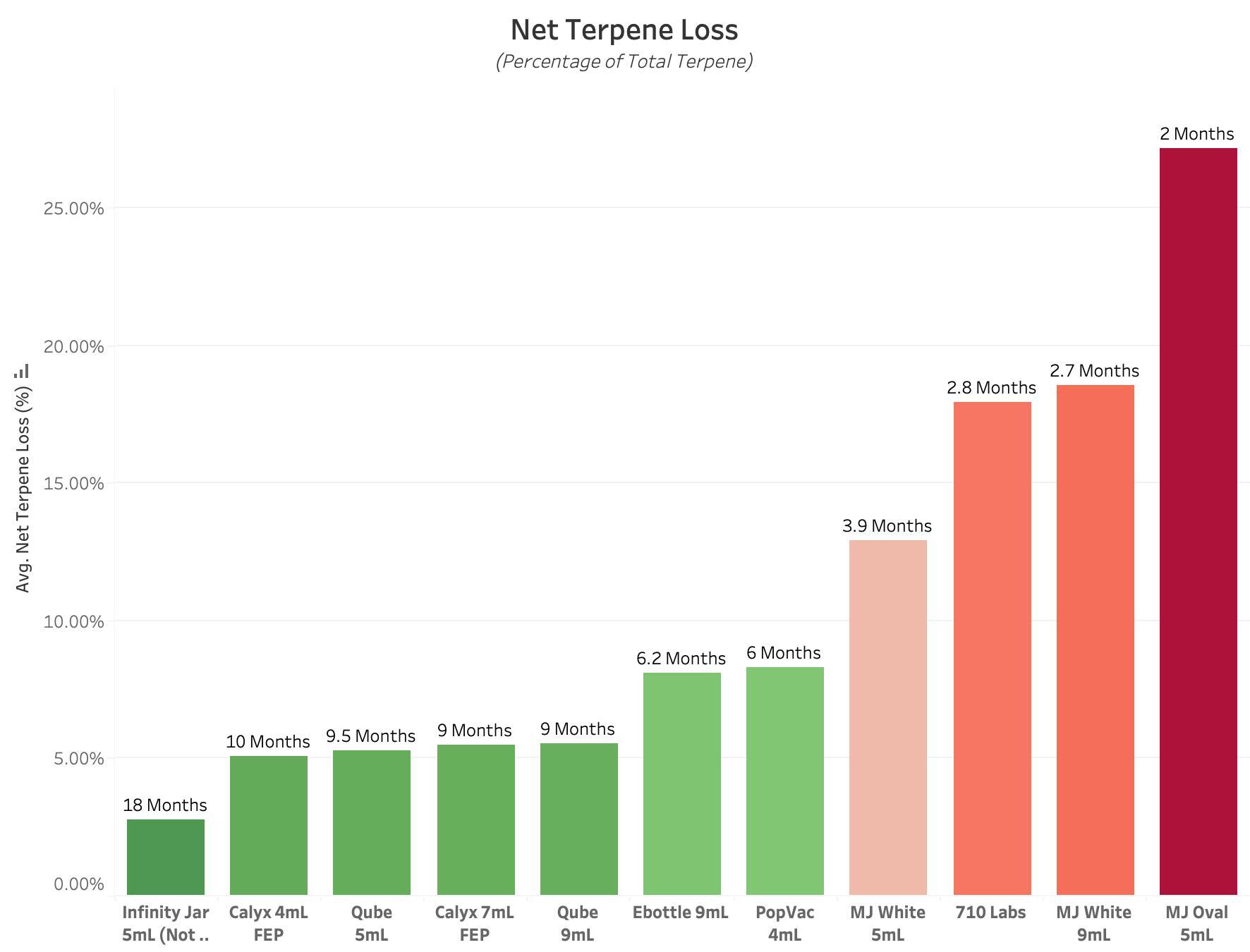
Figure 3 : Graph of Net Terpene Loss for each Experimental Product Type. All values are reported in Percent of Total Terpene Concentration, with lower values equating to better performance. This value reflects the combination of TVTR and TU performance metrics, to display the relative rates at which packaged terpenes are lost to the environment, or absorbed into container components. Each bar is labeled with the approximate Terpene Shelf Life, defined as the amount of time until all terpenes have been removed from the internal environment, based on Net Terpene Loss over 14 Days.
Discussion & Observations
Defect, Breakage, and Contamination
Each container type and sample was thoroughly investigated to identify breakage, defect, or contamination which may negatively impact the results of this test. Of the 11 samples analyzed, 3 samples displayed severe contamination that required washing with soap and water to fully remove. Marijuana Packaging Glossy White 9mL, Marijuana Packaging Glossy White 5mL, and Marijuana Packaging Oval 5mL all contained oil / grease and debris contamination that required this additional cleaning step. These containers were left overnight to fully dry after washing, and were dried by hand the following day to ensure no water remained.
Each container type was also qualitatively analyzed for their ease of opening, and ease of sealing. The standard Ebottles Palm & Turn 9mL exhibited the most difficulty in sealing and access, with some samples requiring excessive force to engage the Child-Resistant Mechanism. Only samples with normal opening and sealing functionality were utilized for this test.
Filling Capacity Variation
Additionally, the standard deviation of filling capacity was captured for all container types, to determine general variation in part-to-part volume. The container that displayed the lowest variability across 5 samples was 710 Labs, with a part-to-part standard deviation of only 0.085mL. The container that displayed the highest variability across 5 samples was PopVac, with a part-to-part standard deviation of 0.612mL.
Terpene Exposure Degradation
Upon completion of the 14 Day Test, several container types exhibited visually apparent degradation of non-glass component materials. Marijuana Packaging Oval 5mL, Marijuana Packaging Glossy White 9mL, and Marijuana Packaging Glossy White 5mL exhibited significant swelling and deformation of their liner material, with some samples exhibiting near total loss of liner shape and functionality. The low resistance of these materials to terpene exposure and degradation likely makes them less effective at packaging cannabis products with high terpene concentration. PopVac containers also experienced degradation of their rubberized “gasket”, with visible separation of the gasket material from the metal disc. This is apparent in the data collected, especially in regards to the good performance in TVTR, but relatively high rates of Terpene Uptake.
Frequently Asked Questions
What is TVTR?
TVTR, or Terpene Vapor Transmission Rate, is a metric that describes how quickly Terpenes escape from a sealed package. This metric is typically reported in % per Year. The lower this value, the better the performance, with a score of 100% per Year indicating a 12 Month Shelf-Life.
Why use Terpene Vapor Transmission Rate?
While TVTR only quantifies one performance attribute of cannabis packaging, it can provide useful insight for producers who specialize in making terpene-rich concentrate products. While TVTR values can provide insight, the test alone ignores another key attribute of cannabis packaging: Terpene Uptake.
What is TU?
TU, or Terpene Uptake, is a metric that describes the percentage of packaged terpenes (distinct from environmental loss, or TVTR) that are absorbed into container materials. This metric is typically reported in a raw percentage, and provides insight into the benefits of different plastic and rubber materials in a cannabis storage environment. The lower this value, the better the performance.
Why use Terpene Uptake?
The vast majority of cannabis packaging utilizes at least one component which contains plastics and / or rubbers, both of which have a high propensity to sequester terpenes away from packaged product. Since TVTR analyzes a complete packaging product for weight loss over time, it will not provide any information about the relative rate of Terpene Uptake for each of the container components and materials. Therefore, it is highly recommended to perform Terpene Uptake and TVTR Testing in parallel to gain the most accurate understanding of a container’s ability to maintain a packaged product’s terpene concentration over time.
Why use Isolated Terpenes as opposed to other VOCs?
VOCs, or Volatile Organic Compounds, describe a wide class of compounds that have high vapor pressure and low water solubility. Some well-known VOCs include Gasoline, Propane, Butane, Formaldehyde, Ethanol, Isopropyl Alcohol, Terpenes, Acetone, etc. While all of these compounds are volatile and organic, they are not equal. Each of these compounds can act as solvents on, and degrade, different materials depending on their chemical composition. Certain materials that are highly resistant to aliphatic hydrocarbons may be chemically weak to alcohols. Due to these differences, it is ineffective to use an alternative VOC to investigate the impact of different packaging environments on Terpene Preservation.
Why are Experimental Containers filled to Maximum Filling Capacity?
Due to the wide variety in size, shape, and closure mechanism for cannabis concentrate containers, a variable filling method makes it impossible to truly understand the isolated performance of each container’s seal. Additionally, many containers have a “True Volume” that varies from the “Listed Volume”, where a 5mL container may have a filling volume of 4.5 - 5.5mL. In order to avoid mis-measurement because of this manufacturing variation, all containers are filled to the same relative volume (Maximum Filling Capacity).
Final Notes
If you have any additional questions regarding the methodology or results of this test, please do not hesitate to reach out to the Calyx Containers team. If you feel that an additional container type should have been included in this test, or if you would like us to perform alternative test methods on Calyx or Competitor packaging products, please contact your Sales Representative. We look forward to your individual analysis of these results, and are excited to continue the conversation on Cannabis Product Testing!


